Ischemia Risk Calculator
Your Genetic Risk Assessment
This tool estimates your genetic contribution to ischemia risk based on key genes discussed in the article. Note: This is not medical advice. Consult your doctor for personalized health recommendations.
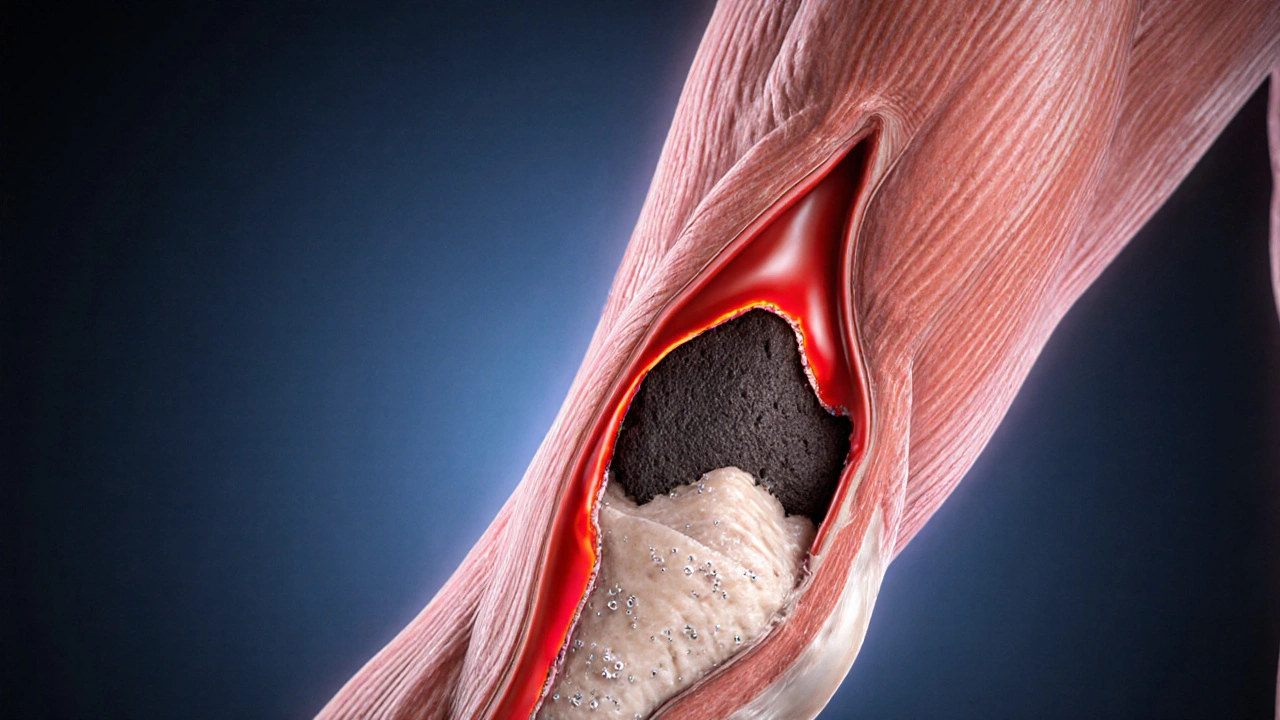
When blood can’t reach a part of your body, that tissue starts to suffer. Ischemia is a condition where reduced blood flow deprives cells of oxygen, often leading to pain, organ damage, or even a heart attack. While lifestyle choices like smoking or high‑fat diets are well‑known triggers, your DNA also plays a quiet but powerful role. In this guide we’ll unpack what the science says, point out the genes that matter most, and show you practical steps to keep your risk in check.
Why genetics matter for ischemia
Think of your genome as a set of instruction manuals for how your heart and blood vessels develop, repair themselves, and respond to stress. Certain manuals contain typo‑like errors-called genetic variants-that can make blood‑vessel walls stiffer, encourage plaque buildup, or impair the body’s ability to form new vessels when needed.
Research over the past decade has linked these variants to a higher likelihood of atherosclerosis, the main culprit behind most ischemic events. In simple terms, if your genes nudge you toward faster plaque formation, the odds of a blockage rise even if you do everything right on the lifestyle front.
Key genes and what they do
Not every gene is a game‑changer, but a handful consistently show up in large studies. Below is a quick rundown of the most influential players.
- APOE - Theε4 version raises LDL cholesterol and is linked to earlier arterial plaque.
- PCSK9 - Certain loss‑of‑function mutations actually lower cholesterol, while gain‑of‑function variants do the opposite.
- MTHFR - A common SNP (C677T) can raise homocysteine, a molecule that damages blood‑vessel lining.
- NOS3 - Variants affect nitric oxide production, influencing vessel dilation.
- KIF6 - TheTrp719Arg variant has been debated, but some data tie it to higher coronary events.
How big is the genetic contribution?
Scientists estimate that genetics accounts for roughly 40‑50% of the variability in ischemic disease risk. The rest comes from diet, exercise, smoking, stress, and other environmental factors. In practice, this means that even if you have a high‑risk gene profile, healthy habits can still offset a sizable portion of that risk.
| Gene | Key Variant | Risk Impact | Population Prevalence |
|---|---|---|---|
| APOE | ε4 allele | 1.8‑fold higher risk of coronary plaque | ~15% worldwide |
| PCSK9 | Gain‑of‑function mutations | 2‑3‑fold increase in LDL‑C | ~1‑2% (rare) |
| MTHFR | C677T (TT homozygote) | ~1.3‑fold higher homocysteine levels | ~10‑12% |
| NOS3 | Glu298Asp | Reduced nitric oxide, modest rise in hypertension | ~30% |
| KIF6 | Trp719Arg | Potential 1.2‑fold rise in myocardial infarction | ~45% |
Family history vs. DNA test: what’s the difference?
Most doctors start with a simple question: “Do any close relatives have heart attacks or strokes?” That’s family history, a quick proxy for shared genetics and lifestyle. However, a family history can be vague-people may not know exact ages of onset or may have missing information.
Enter genetic testing. A saliva or blood sample is analyzed for the variants listed above and many more. The result is a personalized risk score that can be combined with traditional risk calculators (like the Framingham score) for a fuller picture.
Testing isn’t mandatory for everyone. It’s most useful when you have a strong family history, early‑onset disease in relatives, or if you’re considering preventive medication (e.g., statins) at a younger age.
Practical steps if you carry a risk‑gene
- Know your numbers. Get your cholesterol, blood pressure, and fasting glucose checked at least annually. If you have a high‑risk genotype, aim for tighter targets (e.g., LDL‑C <70mg/dL).
- Adopt heart‑healthy habits. A Mediterranean‑style diet, regular aerobic exercise, and quitting smoking can blunt genetic risk by up to 40% according to recent cohort studies.
- Consider medication early. If your PCSK9 mutation is elevating LDL‑C, a PCSK9 inhibitor may be recommended even if your LDL‑C isn’t sky‑high yet.
- Talk to a genetic counselor. They can interpret results, explain inheritance patterns, and guide testing for relatives.
- Track lifestyle interactions. Keep a simple log of exercise minutes, diet quality, and stress levels. Over time you’ll see which habits swing your biomarkers the most.
Remember, a gene isn’t a destiny. It’s a signal that tells you where to focus your preventive effort.
When to get tested and what to expect
Most commercial panels cost between $150‑$400 and return results in 2‑3 weeks. The report usually lists the genes tested, the specific variants found, and a risk interpretation (low, moderate, high). Some labs also include a brief management plan.
If you decide to proceed, here’s a typical workflow:
- Schedule a telehealth or in‑person visit with a primary‑care physician or cardiologist.
- Discuss your family history, personal health goals, and whether a genetic test aligns with those goals.
- Provide a saliva kit (self‑collected) or have a phlebotomist draw blood.
- Wait for the lab to process the DNA and generate the report.
- Review results with a genetic counselor, who will translate technical language into actionable steps.
Insurance coverage varies. In Australia, Medicare may partially cover testing if you meet certain criteria (e.g., early‑onset coronary disease in a first‑degree relative).

Common myths about genetics and ischemia
- Myth: “If I have a risky gene, I’m doomed.”
Reality: Lifestyle changes can offset a large portion of genetic risk. - Myth: “Only men inherit heart‑disease genes.”
Reality: Women inherit the same variants; they often present later, making early awareness critical. - Myth: “Genetic tests are only for research.”
Reality: Clinical labs now offer certified tests that feed directly into medical decision‑making.
Wrap‑up: turning knowledge into action
Understanding the role of genetics in ischemia gives you a clearer map of where risk hides. By combining a family‑history interview, a targeted DNA test, and disciplined heart‑healthy habits, you can steer your own outcomes.
So, if you’ve ever wondered why heart disease runs in your family, the answer likely involves both shared genes and shared kitchens. The good news? You control the kitchen.
Frequently Asked Questions
Can a single gene cause ischemia?
Most cases involve multiple genes plus lifestyle factors. Rare monogenic disorders (like familial hypercholesterolemia) dramatically raise risk, but they represent a small slice of all ischemic disease.
Is genetic testing covered by insurance in Australia?
Medicare may subsidize testing if you meet criteria such as early‑onset coronary disease in a first‑degree relative. Private health funds often cover a portion, especially when a doctor recommends testing for preventive care.
How often should I repeat a genetic test?
Your DNA doesn’t change, so retesting isn’t needed unless new, clinically relevant variants are discovered and added to testing panels. However, you should repeat cardiovascular risk assessments (blood pressure, lipids) regularly.
Do lifestyle changes work for people with high‑risk genes?
Yes. Studies show that a Mediterranean diet, regular exercise, and smoking cessation can reduce the relative risk associated with high‑risk alleles by up to 40%.
What is a SNP and why does it matter?
A single nucleotide polymorphism (SNP) is a one‑letter change in the DNA sequence. Certain SNPs alter how proteins work, influencing cholesterol metabolism, blood‑vessel health, or blood clotting - all key to ischemia risk.

Chuck Bradshaw
October 11, 2025 AT 21:04The relationship between genetic variants and ischemic disease has been mapped through large‑scale genome‑wide association studies. Variants in APOE, PCSK9, MTHFR, NOS3, and KIF6 each contribute measurable shifts in lipid metabolism, homocysteine levels, or endothelial function. For example, carrying one ε4 allele of APOE can increase LDL cholesterol by roughly 15 percent and accelerate plaque deposition. Gain‑of‑function mutations in PCSK9 raise circulating LDL‑C up to threefold, which directly translates into arterial wall burden. The MTHFR C677T homozygous genotype elevates plasma homocysteine, a known pro‑thrombotic agent that damages the intimal layer. NOS3 Glu298Asp reduces nitric oxide bioavailability, limiting vasodilation during periods of increased cardiac demand. KIF6 Trp719Arg, while modest, has been associated with a 20 percent higher odds of myocardial infarction in meta‑analyses. Epidemiological models estimate that these genetic factors explain roughly forty to fifty percent of inter‑individual variance in ischemic risk. The remaining variability is driven by modifiable exposures such as diet, physical activity, smoking, and stress. Consequently, even individuals with a high‑risk genetic profile can blunt disease progression by adhering to heart‑healthy behaviors. Routine lipid panels, blood pressure checks, and glucose monitoring become especially critical for genetically predisposed patients. Lifestyle interventions-Mediterranean‑style eating, regular aerobic exercise, and smoking cessation-have been shown to offset up to thirty percent of genetically mediated risk. Clinical guidelines now endorse the use of poly‑genic risk scores alongside traditional calculators to personalize preventive strategies. However, genetic testing should be interpreted by qualified professionals, as penetrance can vary across ethnicities and family histories. In practice, a combined approach that leverages both genetic insight and aggressive risk factor management offers the best protection against ischemic events.
Howard Mcintosh
October 15, 2025 AT 05:46That article really breaks down the nitty‑gritty of how our DNA can tip the scales on heart health. It’s wild how a single gene tweak can jack up your LDL by a chunk. If you’re carryin’ an APOE‑ε4, just know you might need to keep a closer eye on your diet. And don’t forget to get your blood work checked at least once a year, even if you feel fine. Stay on top of it, definately worth the effort.
Jeremy Laporte
October 18, 2025 AT 03:13Appreciate the deep dive, especially the clear link between homocysteine and vessel health. For anyone reading, remember that supplementing folate and B12 can help lower homocysteine levels, which is a simple step beyond just meds. It’s also cool that the piece mentions how lifestyle can offset a good chunk of genetic risk – that’s empowering. Let’s keep sharing practical tips so the info moves from theory to daily action.
Andy Lombardozzi
October 21, 2025 AT 00:40Genetics set the stage, but diet and exercise are the real directors. The PCSK9 data is especially striking; those rare gain‑of‑function mutations can push LDL sky‑high. If you suspect a family history, talking to a cardiologist about a possible panel can clarify your risk. Meanwhile, a Mediterranean diet rich in omega‑3s and regular cardio are solid defenses.
Joshua Ardoin
October 23, 2025 AT 22:06All this science is fascinating 🌟! Knowing your genetic makeup can feel like holding a cheat‑code for heart health. 🎮💓 If you get a high‑risk score, don’t panic – just double down on veggies, nuts, and daily walks. And hey, a quick DNA test can give you that extra peace of mind. 🚀
Glenn Gould
October 26, 2025 AT 18:33Totally agree with the vibe – a DNA test is like a sneak peek at your future health. If the results show a risk, just treat it like a heads‑up and bump up the cardio sessions. Also, try to cut out processed snacks; they mess with LDL big time.
Poonam Sharma
October 29, 2025 AT 16:00In a country where heart disease is the leading killer, this genetic primer is a national imperative! The jargon may be dense, but the implication is crystal clear: we must invest in population‑wide screening programs, especially for high‑risk alleles like APOE‑ε4. Ignoring this data is tantamount to negligence, and that is unacceptable for the health of our citizens.
Meigan Chiu
November 1, 2025 AT 13:26While the enthusiasm for mass screening is admirable, one must caution against over‑interpretation of modest risk alleles. The KIF6 Trp719Arg variant, for instance, shows only a marginal effect size and has been contested in recent meta‑analyses. Therefore, policies should prioritize high‑impact mutations like PCSK9 gain‑of‑function, which have clear therapeutic pathways. Moreover, any recommendation must be accompanied by rigorous cost‑benefit analyses.
Patricia Hicks
November 4, 2025 AT 10:53Reading through the breakdown of each gene really puts into perspective how layered our cardiovascular risk truly is. The APOE ε4 discussion reminded me of the importance of lipid profiling from an early age, especially for those with a family history of early heart attacks. I also love that the article emphasizes the 40‑50% genetic contribution, which reinforces that we still have a huge window to intervene through lifestyle. In my own experience, adopting a plant‑forward diet and consistent moderate‑intensity exercise dramatically improved my lipid panel, despite a modest APOE risk. It's also worth noting that regular stress‑reduction practices, like mindfulness, can indirectly influence endothelial function, tying back to the NOS3 variant impact. For anyone considering genetic testing, coupling the results with a personalized wellness plan makes the data actionable rather than merely informational. Remember, knowledge is power, but action is the real game‑changer. Keep sharing these insights so more people can take charge of their heart health!
Quiana Huff
November 7, 2025 AT 08:20Super useful breakdown! I’m especially excited about the potential of PCSK9 inhibitors for those with gain‑of‑function mutations 🚀. For anyone who’s skeptical, just remember that these drugs have already shown a 50‑% reduction in major cardiovascular events in high‑risk trials. Pair that with a low‑carb, high‑fiber diet and you’ve got a solid defense. 🙌
William Nonnemacher
November 10, 2025 AT 05:46Genetics only tells part of the story.
Alex Ramos
November 13, 2025 AT 03:13Indeed, the integration of poly‑genic risk scores, alongside traditional metrics such as systolic blood pressure, total cholesterol, and smoking status, creates a multidimensional risk matrix that clinicians can employ; however, the implementation must consider population stratification, allelic frequency variance, and the ethical implications of predictive health data, which, if mishandled, could exacerbate health disparities; consequently, standardized reporting frameworks, transparent algorithms, and robust patient education are essential components of any genomic‑driven preventive strategy; moreover, healthcare systems should allocate resources for genetic counseling services to interpret these complex results, ensuring that individuals receive personalized, evidence‑based recommendations that align with their unique genetic architecture and environmental exposures.
Mita Son
November 16, 2025 AT 00:40Whoa, that over‑the‑top sentence just blew my mind – gotta love how the data can get super technical! But seriously, the gist is that genetic testing isn’t a magic bullet; it’s a tool, and you still need to eat right and move around. And btw, those fancy terms? They’re just a fancy way of saying “watch your heart”.
ariel javier
November 18, 2025 AT 22:06While the article provides a concise overview, it insufficiently addresses the heterogeneity of allele penetrance across diverse ethnic cohorts. A more rigorous analysis, complete with confidence intervals and hazard ratios, would better inform clinicians of the true incremental value of genetic screening beyond conventional risk calculators.
Bryan L
November 21, 2025 AT 19:33Great points raised. It’s reassuring to see the emphasis on combining genetic insight with lifestyle changes. 😊 Let’s keep the conversation going and help each other stay proactive about heart health.