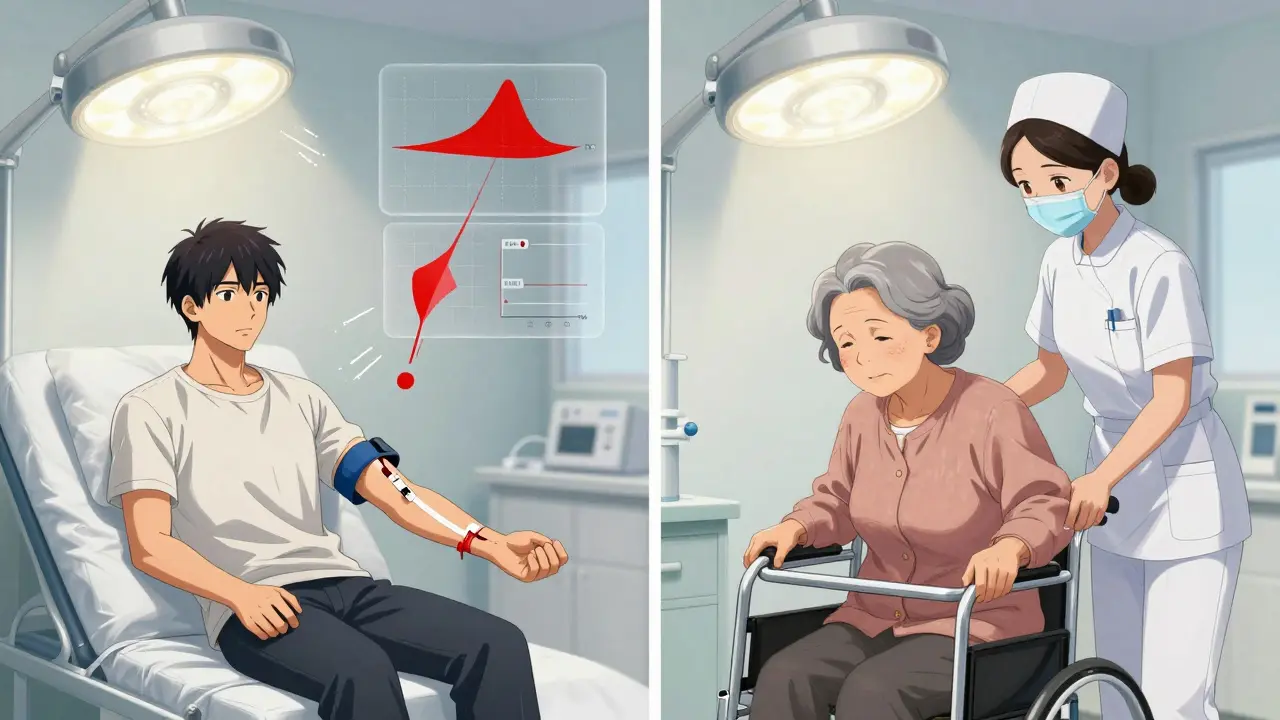
When a generic drug hits the shelf, you assume it works just like the brand-name version. But here’s the thing: most bioequivalence studies used to test that assumption were done on young, healthy men. That’s not how most people actually take these drugs. Women, older adults, and people with different body types are often left out of the equation-despite the fact that their bodies process medicine differently. This isn’t just an oversight. It’s a gap in safety and effectiveness that regulators are finally starting to fix.
What Bioequivalence Really Means
Bioequivalence means two drugs-say, a brand-name pill and its generic copy-deliver the same amount of active ingredient into your bloodstream at the same speed. If they don’t, you might get too little of the drug and it won’t work, or too much and you risk side effects. The standard test is a crossover study: one group takes the brand drug first, then the generic, and vice versa, with blood samples taken over time to measure absorption.
For decades, these studies relied on a narrow group: men aged 18 to 40, with normal weight, no chronic conditions, and no medications. Why? Because it was easier. Their bodies were predictable. But that predictability came at a cost. It ignored the fact that sex and age change how drugs are absorbed, distributed, metabolized, and cleared.
Why Sex Matters in Bioequivalence
Women aren’t just smaller versions of men. Their bodies handle drugs differently. Hormones, body fat percentage, liver enzyme activity, and even stomach emptying rates vary. For example, some drugs like certain antidepressants and statins are cleared faster in men, while others like warfarin are processed slower in women. In one 2018 study, a generic version of a common heart medication showed a 79% point estimate of bioavailability in men-but 95% in women. At first glance, that looked like a problem. But when the study was repeated with more participants, the difference vanished. The initial result? A statistical fluke caused by too few women in the trial.
The U.S. Food and Drug Administration (FDA) now requires that if a drug is meant for both men and women, the study must include roughly equal numbers of each. That’s new. Before 2023, the FDA just suggested it. Now, sponsors must justify why they’d exclude one sex. The European Medicines Agency (EMA) still says subjects “could belong to either sex,” leaving room for imbalance. Brazil’s ANVISA, however, demands exact 50-50 splits. The inconsistency creates headaches for drug makers trying to sell globally.
And it’s not just about fairness. Women are more likely to experience adverse reactions. A 2021 FDA analysis of 1,200 generic drug applications found that only 38% of studies met the 40-60% female participation threshold. The median? Just 32%. That’s a problem when you consider that 63% of people taking levothyroxine for thyroid disease are women. If the bioequivalence study was done mostly on men, how do we know the generic works the same for them?
Age Isn’t Just a Number
Older adults are another group often missing from bioequivalence trials. As we age, our kidneys and liver slow down. Blood flow changes. Muscle mass drops. All of this affects how drugs move through the body. A drug that’s safe and effective in a 25-year-old might build up to toxic levels in a 70-year-old.
The FDA now says: if your drug is meant for older patients, include people aged 60 and up. Or explain why you didn’t. The EMA doesn’t require it. ANVISA caps the upper age limit at 50. That’s a red flag. Many chronic conditions-hypertension, diabetes, arthritis-are more common in people over 60. Yet most bioequivalence studies still exclude them.
Here’s the catch: older adults are harder to recruit. They’re more likely to have other health issues, take multiple medications, or have mobility problems. That means studies cost more and take longer. But skipping them isn’t an option. A 2022 study found that a generic version of a blood thinner showed a 20% higher peak concentration in elderly volunteers compared to younger ones-something the original trial missed because it only tested people under 50.
Regulatory Differences Around the World
Not every agency sees this the same way. The FDA is pushing hardest for inclusion. Their 2023 draft guidance is the strictest yet: balanced sex representation is the default. The EMA is cautious, focused on detecting formulation differences, not reflecting real-world populations. ANVISA is the most rigid, requiring exact age and sex splits. Health Canada falls somewhere in between, allowing ages 18 to 55.
This patchwork creates real challenges for drug manufacturers. A company designing a study for the U.S. market might include older adults and women. But if they want to sell in Europe or Brazil, they have to run separate studies-or risk rejection. It’s inefficient. It’s expensive. And it delays access to affordable medicines.
Why It’s Hard to Get It Right
Even when regulators demand balance, it’s tough to deliver. Recruiting women into clinical trials remains harder than recruiting men. Many women juggle caregiving, work, or family responsibilities. Others worry about risks during childbearing years-even if they’re using contraception. Sites report recruitment for gender-balanced studies takes 40% longer.
Statistical power is another issue. Small studies (under 24 participants) can’t reliably detect sex-based differences. One study with just 12 people showed a dramatic difference between sexes. A follow-up with 36 participants showed none. The lesson? Bigger isn’t just better-it’s necessary.
Companies are starting to adapt. A 2022 survey found 68% of contract research organizations now use targeted outreach to recruit women. Some offer flexible hours, childcare support, or transportation. But only 29% track sex-specific pharmacokinetic data routinely. That means even when women are in the study, we’re not always analyzing the results by sex.

What’s Next for Bioequivalence
The future is clear: bioequivalence studies need to reflect real patients. The FDA’s 2023-2027 plan calls this a top priority. The EMA is reviewing its 2010 guidelines and may update them soon. Researchers at the University of Toronto found that 37% of commonly tested drugs are cleared 15-22% faster in men than in women. That’s not noise-it’s biology.
Experts are now talking about sex-specific bioequivalence criteria, especially for drugs with narrow therapeutic windows-like warfarin, lithium, or digoxin. One wrong dose can be dangerous. If women clear the drug slower, shouldn’t the acceptable range be adjusted?
It’s not just about science. It’s about justice. People deserve medicines that work for them, not just for the average young man. The old model served its purpose when we knew less. But now we know better. And regulators are finally catching up.
What This Means for You
If you’re taking a generic drug-especially if you’re a woman over 50-you should know this: the study that proved it was “equivalent” may not have included people like you. That doesn’t mean it won’t work. But it means the evidence isn’t as strong as it could be.
Ask your pharmacist or doctor: “Was this generic tested on people my age and sex?” If they don’t know, it’s a sign the system still has gaps. You’re not asking for special treatment. You’re asking for the same level of safety everyone else got.
The science is evolving. The regulations are catching up. And the patients? We’re finally being seen.
Why were bioequivalence studies historically done only on young men?
Early bioequivalence studies used young, healthy men because their physiology was seen as more predictable and easier to control. Hormonal fluctuations in women, age-related changes, and chronic conditions were viewed as “noise” that could obscure formulation differences. It was a practical shortcut, not a scientific necessity-and one that ignored real-world patient diversity.
Do women and men metabolize drugs differently?
Yes. Women often have higher body fat, lower muscle mass, slower gastric emptying, and differences in liver enzyme activity-all of which affect how drugs are absorbed and cleared. For example, some antidepressants and statins are metabolized faster in men, while drugs like warfarin and benzodiazepines can reach higher concentrations in women, increasing the risk of side effects.
Are older adults included in bioequivalence studies today?
It depends on the regulator and the drug. The FDA now requires inclusion of adults aged 60+ if the drug targets older patients, or a strong justification for exclusion. The EMA doesn’t mandate it. ANVISA caps participation at age 50. This means many older adults still aren’t represented in the data used to approve generics they’ll be prescribed.
What’s the minimum number of participants needed in a bioequivalence study?
The EMA requires at least 12 evaluable subjects, but most studies enroll 24 to 36 to account for dropouts and ensure enough power to detect differences-especially when analyzing subgroups like sex or age. Studies with fewer than 24 participants often lack statistical reliability for detecting real-world variations.
Why does the FDA now require balanced sex representation?
Because historical data showed that drugs approved based on male-only studies sometimes caused unexpected side effects or reduced effectiveness in women. The FDA’s 2023 draft guidance recognizes that bioequivalence must reflect the actual patient population-not just the easiest test group. If a drug is used by both men and women, the study should too.
Can bioequivalence results from young adults be applied to older people?
Not reliably. Age changes how the body absorbs, distributes, and eliminates drugs. A generic that’s bioequivalent in a 25-year-old may behave differently in a 70-year-old due to reduced kidney or liver function. Regulatory agencies now require specific justification or direct testing in older adults when the drug is intended for them.
What You Can Do
Stay informed. Ask questions. If you’re prescribed a generic drug and you’re a woman, over 60, or have a chronic condition, don’t assume the study that approved it included people like you. Talk to your pharmacist. Check the drug’s prescribing information. Push for transparency. The system is changing-but it needs pressure to move faster.
Every time a bioequivalence study includes more women, more older adults, more diversity-it doesn’t just make science better. It makes medicine safer for everyone.

Pat Dean
January 17, 2026 AT 08:16This is why America’s healthcare system is broken. They test drugs on 22-year-old guys in gym shorts and then slap a generic label on it for my 68-year-old mom who’s on eight meds. It’s not science-it’s corporate laziness dressed up as regulation.
And don’t get me started on how women are treated like lab rats when they’re fertile and invisible when they’re not. Wake up, FDA. We’re not your control group.
Someone’s gotta pay for this oversight. And it’s always the patient.
Jay Clarke
January 17, 2026 AT 20:06Bro. I just took a generic Adderall last week and felt like a ghost. Then I read this and realized-oh. The study was done on 24 dudes who haven’t slept since 2019. No wonder I felt like my brain was on mute.
They don’t test on people who actually take the drug. They test on people who look like they belong in a pharmaceutical ad. It’s not bioequivalence. It’s bio-illusion.
Selina Warren
January 19, 2026 AT 04:00Let me tell you something. I’m a 54-year-old woman with hypothyroidism. I’ve been on levothyroxine for 17 years. I’ve switched generics five times. Three of them made me feel like I was slowly drowning in molasses.
And guess what? None of the studies that approved those generics had more than 20% women. Not even close.
This isn’t about ‘science.’ It’s about who gets to be considered human. We’ve been treated like afterthoughts since the 1970s. It’s time we stopped being polite and started demanding better.
My body is not a statistical outlier. I am the norm. And I deserve a drug that works for me-not for some 28-year-old male researcher’s idea of ‘ideal.’
Robert Davis
January 20, 2026 AT 04:45Look, I get it. Women metabolize things differently. Older people have slower kidneys. But let’s be real-most of these studies are already underfunded. Adding more variables means more cost, more time, more headaches.
Maybe the real issue isn’t the studies-it’s that we expect one pill to work for everyone. We live in a world of personalized medicine, but we still treat drugs like they’re mass-produced cereal.
It’s not the regulators’ fault. It’s ours. We want cheap generics and perfect results. We can’t have both without more investment.
And honestly? If your drug doesn’t work, switch brands. Pay the extra $5. Your life’s worth it.
Eric Gebeke
January 21, 2026 AT 05:19Oh here we go. The ‘women and old people are different’ narrative again. Let me guess-next they’ll say we need different vaccines for left-handed people or people who eat kale.
These studies are supposed to measure formulation equivalence, not demographic diversity. If your body reacts weirdly to a generic, maybe it’s your metabolism, not the study’s fault.
And don’t even get me started on how people think ‘bioequivalence’ means ‘identical experience.’ It doesn’t. It means the same amount of drug gets in your blood. That’s it.
Stop turning pharmacology into a social justice campaign. Just take your pill and shut up.
Jake Moore
January 21, 2026 AT 21:45As someone who works in clinical research, I can tell you this: the industry is changing, but slowly. The FDA’s 2023 guidance is a game-changer. Companies are finally hiring dedicated recruitment teams just to find older women and people with comorbidities.
We’re seeing more sites offering rideshares, childcare, weekend appointments. It’s not perfect, but it’s progress.
The biggest hurdle? Data analysis. Even when we recruit diverse groups, we don’t always analyze results by sex or age. That’s on us. We’re starting to fix that.
And yes-it costs more. But so does a hospitalization from a bad generic switch. The math is starting to add up.
Joni O
January 23, 2026 AT 05:20my dr just switched me to a new generic for my blood pressure med and i felt dizzy for 3 days… i didn’t know why until i read this. i’m 62 and a woman. the study probably had 1 guy who was 23 and 11 women who were 25. why do we keep doing this?
can someone please tell me how to check what population was used in the study? i want to ask my pharmacist but i don’t even know what to ask.
Ryan Otto
January 24, 2026 AT 01:37Let’s be honest: this isn’t about equity. It’s about control. The FDA, EMA, and other agencies are being manipulated by activist groups pushing a narrative of ‘inclusion’-a euphemism for identity politics. The science has been hijacked.
Biological differences exist, yes. But forcing demographic quotas into clinical trials undermines statistical validity. You can’t design a drug for ‘everybody’ because ‘everybody’ doesn’t exist.
And don’t be fooled by the ‘63% of levothyroxine users are women’ argument. That’s a post-hoc justification, not a scientific principle.
Real science doesn’t care about your gender or age. It cares about data. And data doesn’t lie-until you start cherry-picking demographics to fit your agenda.
Max Sinclair
January 25, 2026 AT 15:13I’ve been reading this thread and honestly? I’m just tired.
We all want safe, effective medicine. But we’re talking past each other. The people who say ‘it’s just about the data’ are ignoring real human suffering. The people who say ‘it’s about justice’ are sometimes ignoring the limits of science.
The truth? We need both.
More diverse trials aren’t a political move-they’re a practical one. If a drug behaves differently in women or older adults, we need to know that before it hits the shelf.
And yes, it costs more. But so does a lawsuit when someone has a stroke because their generic didn’t work the same way.
Let’s stop treating this like a debate and start treating it like a responsibility.
Praseetha Pn
January 26, 2026 AT 01:55OMG this is so obvious and yet NO ONE talks about it! I’m from India and my grandma takes a generic blood thinner and she nearly died last year because her levels spiked. The doctor said it was ‘bioequivalent’ but guess what? The study was done on 18-year-old Indian men who work in call centers and eat spicy food every day.
My grandma is 71, eats rice and dal, has diabetes, and takes 5 other meds. How is that ‘equivalent’? It’s not. It’s a death sentence wrapped in a patent.
And don’t even get me started on how the FDA and EMA are just playing nice with pharma companies while real people die. This is a global scam. They don’t care if you’re old, female, or brown-they just want you to take the pill and shut up.
Nishant Sonuley
January 27, 2026 AT 13:34Look, I get why this is frustrating. I’ve seen the data. I’ve sat in meetings where someone says, ‘We can’t recruit older adults-they’re too complicated.’ And I want to scream. But here’s the thing: complexity isn’t an excuse. It’s a challenge.
What if we redesigned trials to be more patient-centered? What if instead of dragging 30 people into a clinic for 10 days, we did home-based blood draws with telehealth follow-ups? What if we partnered with senior centers and community pharmacies to recruit?
It’s not impossible. It’s just not profitable yet.
But here’s the secret: the patients who’ve been left out? They’re the ones who’ll pay the most for a drug that actually works. They’re not just ‘demographics.’ They’re customers. And they’re not going away.
So maybe the real question isn’t ‘Why don’t we include them?’
It’s ‘Why haven’t we figured out how to profit from doing the right thing yet?’
Emma #########
January 29, 2026 AT 05:17Thank you for writing this. I’ve been too scared to speak up before. I’m 59 and I take a generic antidepressant. I switched to a new brand last year and felt like I was falling apart. I thought it was me. Turns out, the study had 1 woman out of 24 participants.
I didn’t know I could ask about the study population. I thought it was all secret.
I’m going to my pharmacist tomorrow. I’m not asking nicely. I’m demanding to see the data. Because I’m tired of being invisible.
Andrew McLarren
January 30, 2026 AT 01:26The regulatory divergence between the FDA, EMA, and ANVISA reflects a fundamental tension in pharmaceutical governance: standardization versus contextualization. While harmonization remains an ideal, the biological heterogeneity of human populations necessitates jurisdiction-specific parameters.
It is neither scientifically tenable nor ethically defensible to generalize pharmacokinetic parameters derived from homogenous cohorts to heterogeneous patient populations.
Therefore, the adoption of stratified bioequivalence criteria-particularly for drugs with narrow therapeutic indices-is not merely prudent; it is imperative for public health integrity.
Further, the ethical imperative to include underrepresented demographics must be operationalized through incentivized trial design, not punitive mandates.
The path forward lies not in uniformity, but in calibrated, evidence-based inclusivity.
Pat Dean
February 1, 2026 AT 01:17Andrew, you sound like a professor who’s never had to pick up a prescription for someone who’s actually sick.
‘Calibrated inclusivity’? That’s corporate speak for ‘we’ll do the bare minimum and call it progress.’
My mom didn’t die because the science wasn’t ‘calibrated.’ She died because nobody bothered to test the damn drug on people like her.
You can polish your words all you want. The truth is still ugly: the system doesn’t care until someone dies.